What Is the Hybridization of the Se Atom in Sef6
SeF6 Hybridization Hybridization is one of the fundamental concepts used to explain bond formation. If there are two lone pairs of electrons on the central atom they will be 180 apart.

Solved The Hybridization Of Se In Sef6 Is 0 A Sp B Sp C Sp Dsp E Sp
Normally SF6 can be prepared by exposing or combining S8 with F2.

. A What is the hybridization of the Se atom in SeF6 sp3d2 What orbitals make Up the sigma bond between Se and F in SeF. In the case of the Se6 Lewis structure focusing on the hybridization of the central atom is the key. 4 The hybridization that corresponds to five electron pairs is sp³d.
SeF6 Lewis structure hybridization Hybridization is a very important concept in chemical bond formation. Sicl4 hybridization bond angle For which molecules can we not use valence bond theory to explain the bonding. In The Diagrams Below Represents An Electron With Spin 12 And Represents An Electron With Spin -12.
Therefore hybridization state of selenium in is. Hybridization of SF6 Sulfur Hexafluoride The hybridization of SF6 is sp 3 d 2 type. Sp3d2 hybrid orbital on Se and unhybridized p orbital on F What are the approximate bond angles in SeF6.
If I talk about its hybridisation that is sp3d2 Se has 6 valance electron and there are 6 fluorine atomsSo 6626 Total electron pair is 6 and hybridisation is sp3d2. Just to describe the compound in brief Sulphur Hexafluoride is a type of greenhouse gas which is colourless odourless non-toxic and non-flammable. 2 Incubate at 72C for DNA synthesis.
The hybridization of Se in SeF6 is. Since there are 6 bonding pairs around the central selenium atom the electron arrangement of silicon is octahedral. Want this question answered.
Among them 6 are bond pair as there are 6 F participating in bond and 0 lone pain. Two additional Ï bonds. Describe The Hybridization State Of Se In SeF6 By Selecting From The Orbital Diagrams Of Se In Its Excited State Listed Below.
We conclude that the selenium use hybridized orbitals in bonding with fluorine because the orbitals have octahedral arrangement. Spd 26The nitrosyl ion NO has ten bonding electrons and four antibonding electrons. 3 Incubate at 60C for primer hybridization.
There are 6 valence electrons present in the selenium atom. First excited state of sulphur. It is also an inorganic and a non-polar gas.
We conclude that the selenium use hybridized orbitals in bonding with fluorine because the orbitals have octahedral arrangement. It â In ICl5 hybridization of the central iodine atom is sp3d2. First of all SeF6 is octahedral in shape.
1 Incubate at 94C to denature DNA strands. The hybridization of the central atom in NOCl is sp2. Be notified when an answer is posted.
BeCl2 Clâ Beâ Cl bond angle 180 Br2 SF6 Fâ Sâ F bond angles 90 and 180. As the name suggests it is the mixing of 2 orbitals to give rise to a new type of hybridized orbital which has shape energies etc. Similarly it is asked what is the hybridization of the central atom in SeF6.
Since there are 6 bonding pairs around the central selenium atom the electron arrangement of silicon is octahedral. Place the following steps in the PCR procedure in the correct order. While it too is used in explosives it is much less reactive than white phosphorus.
Answer the following questions about SeF6. The nitrogen atom is the central atom and the oxygen atoms are equidistant from it. What is the hybridization about the central atom in OCl2.
The central atom is oxygen with sp3 hybridization. What is the hybridization of the central atom in SeF6. What is the hybridization of Se in SeF6.
1072 The bond angle in P 4 is 60. And geometry is octahedral. What is the hybridization of Se in SeF6.
Besides what is the hybridization of Se in sef6. Hybridization involves mixing two or more atomic orbitals to form orbitals with the same energy shape and size. The hybridized orbitals form bonds and the extent of overlap is better than unhybridized orbitals.

Solved Answer The Following Questions About Sef6 A What Is The Hybridization Of The Se Atom In Sef6 Sp3d2 What Orbitals Make Up The Sigma Bond Between Se And F In Sef
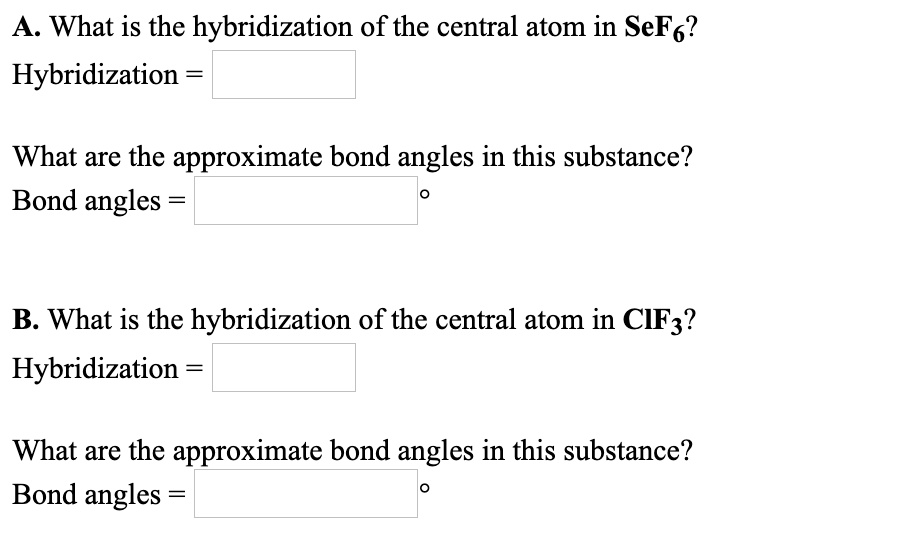
Solved A What Is The Hybridization Of The Central Atom In Sef6 Hybridization What Are The Approximate Bond Angles In This Substance Bond Angles B What Is The Hybridization Of The Central Atom
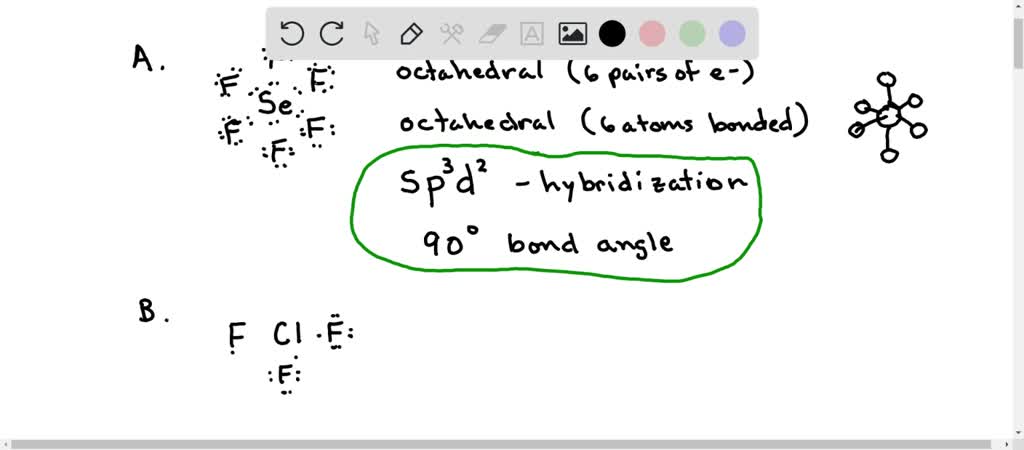
Solved A What Is The Hybridization Of The Central Atom In Sef6 Hybridization What Are The Approximate Bond Angles In This Substance Bond Angles B What Is The Hybridization Of The Central Atom

Sef6 Lewis Structure Geometry Hybridization And Polarity Techiescientist
Comments
Post a Comment